Ich E6 Describes Standards That Apply to
As such they are not subject to enforcement by US. The Code of Federal Regulations.
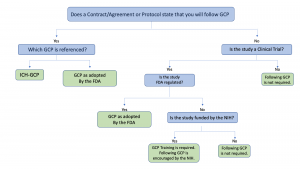
Application Of Good Clinical Practice At Uw Madison Research Uw Madison
Emphasis added See ICH E6 441 This language recommends but does not require such review.

. The FDA will apply. In the United States following the ICH E6 GCP is. Voluntary for FDA-regulated drug studies.
The ICH E6 GCP describes standards that apply to. In general the ICH Good Clinical Practice guidelines are recommendations not legal requirements. Sets with similar terms.
Investigators sponsors and IRBs.

Are Clinical Trial Teams Prepared For Ich E6 R3

Are Clinical Trial Teams Prepared For Ich E6 R3


Comments
Post a Comment